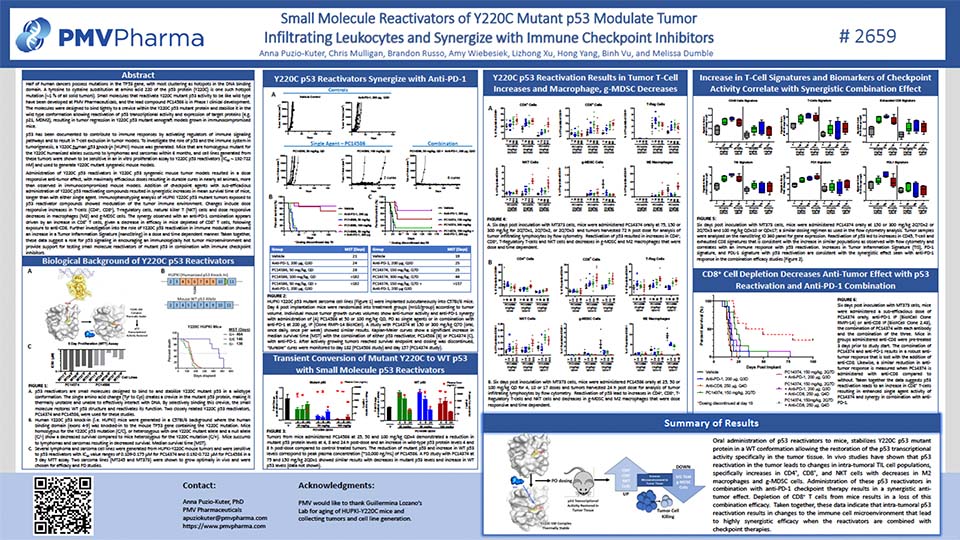
The TP53 Y220C mutation has been identified in many types of solid tumors
The TP53 Y220C mutation is a potential treatment target in cancer and has been identified in over 30 different solid tumor types.1–5 Under normal conditions, p53 is a tumor-suppressor protein and has a key role in disrupting tumor progression.6,7 The mutated p53 Y220C protein forms a small pocket on the surface of the p53 protein, which causes the protein to lose its structural shape; this results in a loss of function, and can lead to tumor progression.1–3,8
References: 1. Blanden AR, et al. Drug Discov Today. 2015;20:1391–1397; 2. Baud MGJ, et al. Eur J Med Chem. 2018;152:101–114; 3. Tang Y, et al. J Phys Chem B. 2021;125:10138–10148; 4. FoundationInsights™. A proprietary database used under license with review and approval from Foundation Medicine®. Available at: https://www.foundationmedicine.com/service/genomic-data-solutions. Accessed May 2024; 5. Schram A, et al. Poster presentation at AACR-NCI-EORTC 2023, Boston, USA. October 11–15, 2023; 6. Kastenhuber ER, et al. Cell. 2017;170:1062–1078; 7. Blagih J, et al. J Cell Sci. 2020;133:jcs237453; 8. Baugh EH, et al. Cell Death Differ. 2018;25:154–160.
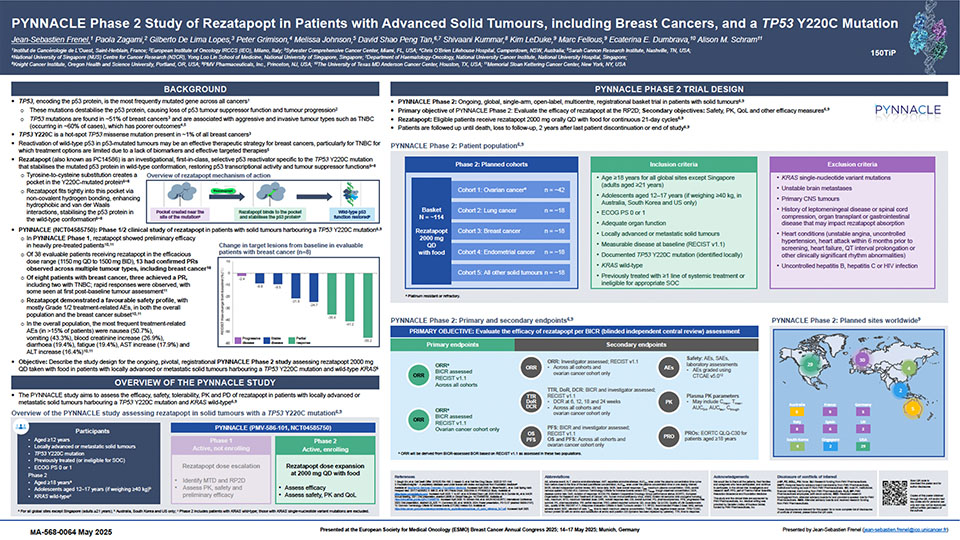
Rezatapopt (also known as PC14586) is being investigated in TP53 Y220C cancer
Rezatapopt is a first-in-class, selective p53 reactivator specific to the mutated p53 Y220C protein.1–4 Rezatapopt is currently under investigation for the treatment of patients with locally advanced or metastatic solid tumors that have a TP53 Y220C mutation (or TP53 Y220C cancer), including but not limited to ovarian cancer, lung cancer, breast cancer, and endometrial cancer.1–4
References: 1. ClinicalTrials. gov. NCT04585750. The evaluation of PC14586 in patients with advanced solid tumors harboring a p53 Y220C mutation. Available at: https://clinicaltrials.gov/study/NCT04585750. Accessed September 2024; 2. Dumble M, et al. Oral presentation at AACR 2021, Virtual. April 10–15, 2021; 3. Dumbrava EE, et al. Oral presentation at ASCO 2022, Chicago, USA. June 3–7, 2022; 4. Schram A, et al. Poster presentation at AACR-NCI-EORTC 2023, Boston, USA. October 11–15, 2023.
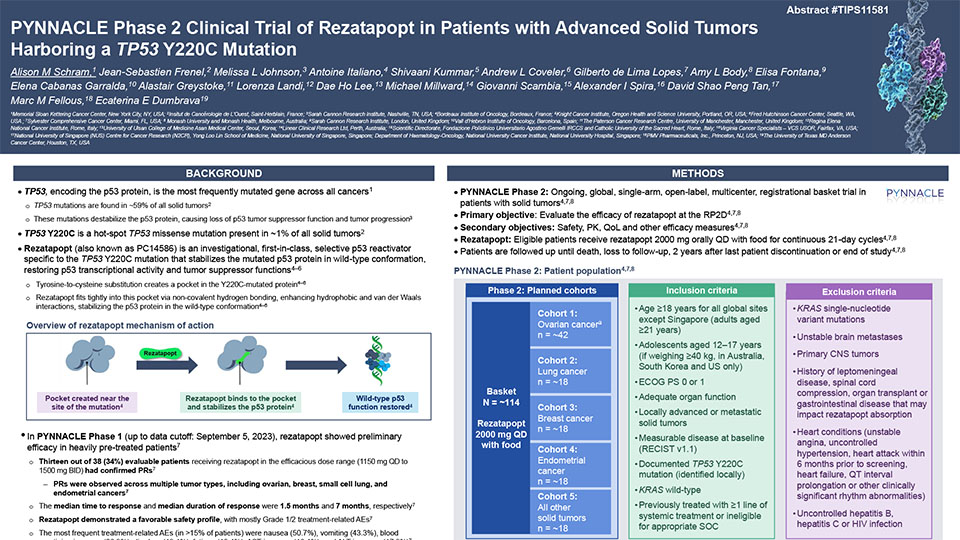
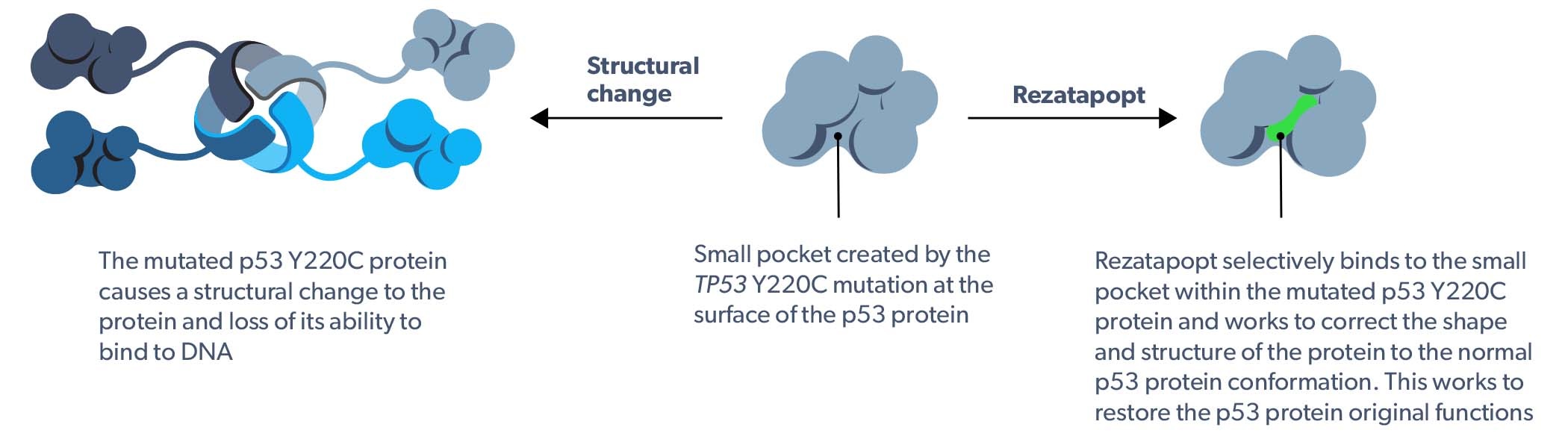
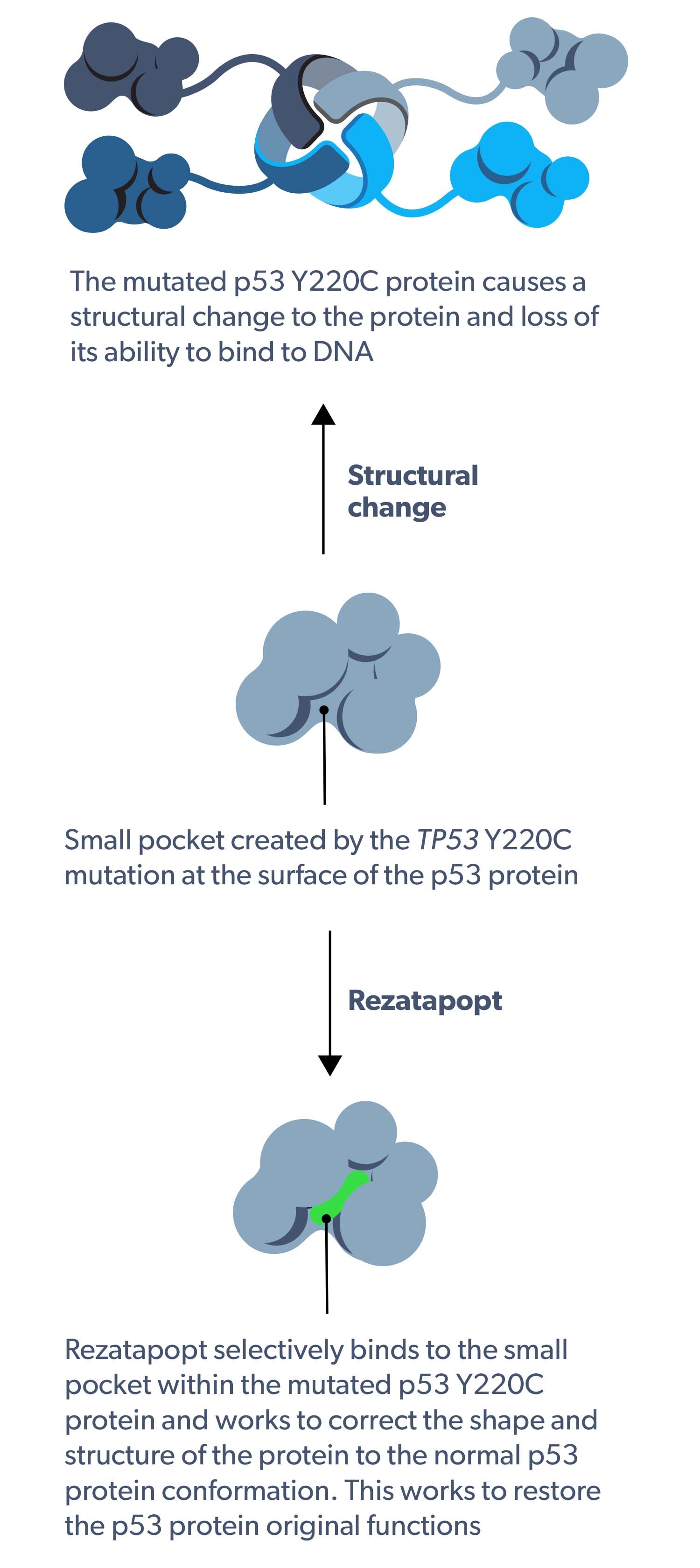
Rezatapopt selectively reactivates p53 Y220C
Rezatapopt selectively binds to the small pocket within the TP53 Y220C mutated protein and works to correct the shape and structure of the protein to normal p53 protein conformation.1–4 This works to restore the p53 protein original functions, including the ability to disrupt tumor progression.1–4
Rezatapopt mechanism of action
References: 1. ClinicalTrials. gov. NCT04585750. The evaluation of PC14586 in patients with advanced solid tumors harboring a p53 Y220C mutation. Available at: https://clinicaltrials.gov/study/NCT04585750. Accessed September 2024; 2. Dumble M, et al. Oral presentation at AACR 2021, Virtual. April 10–15, 2021; 3. Dumbrava EE, et al. Oral presentation at ASCO 2022, Chicago, USA. June 3–7, 2022; 4. Schram A, et al. Poster presentation at AACR-NCI-EORTC 2023, Boston, USA. October 11–15, 2023.
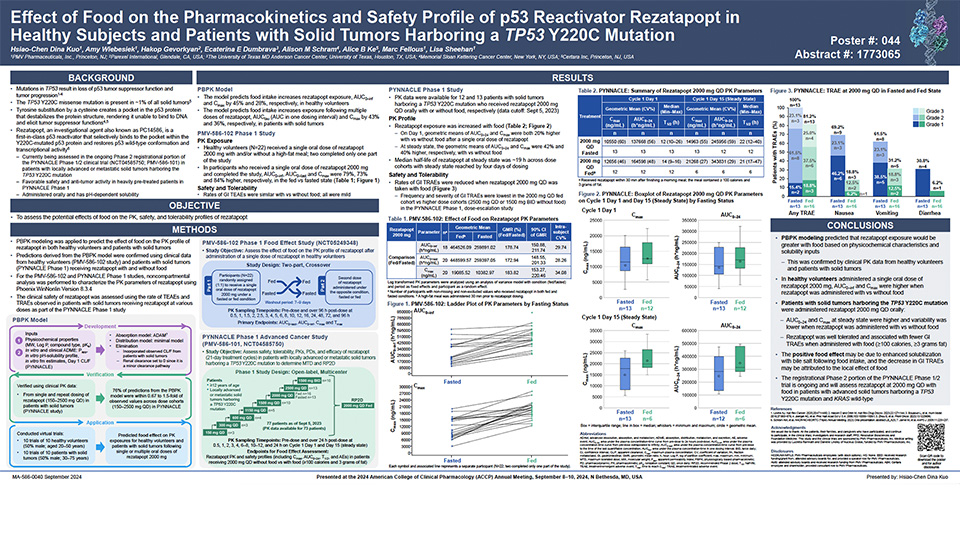
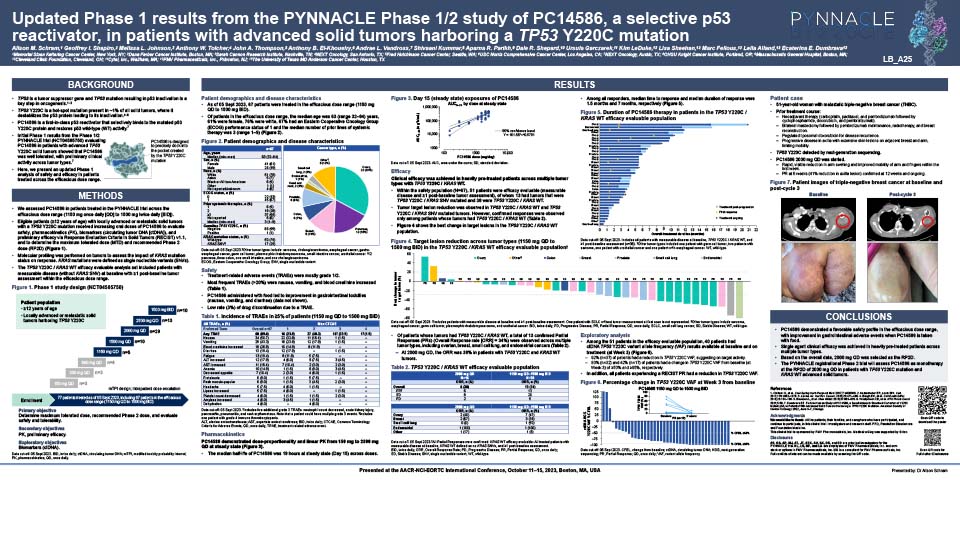
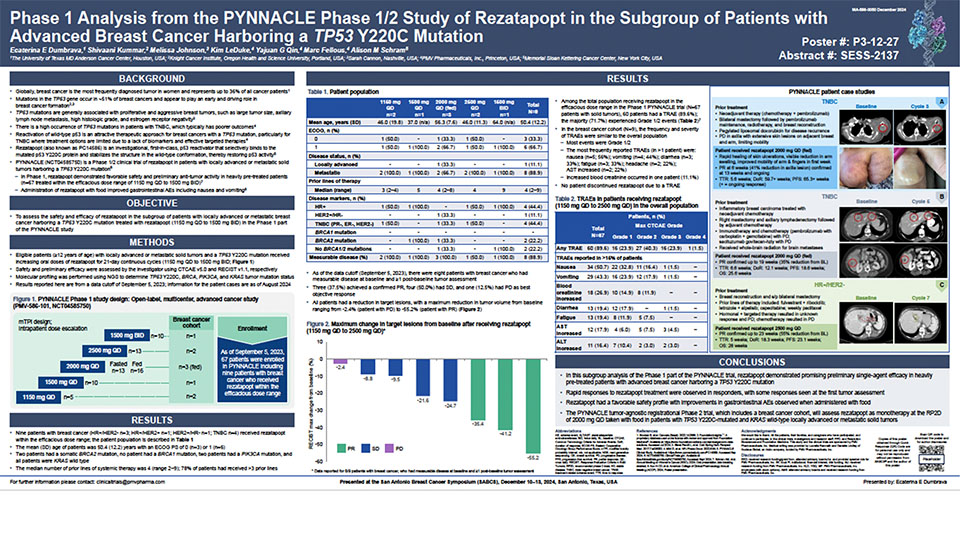
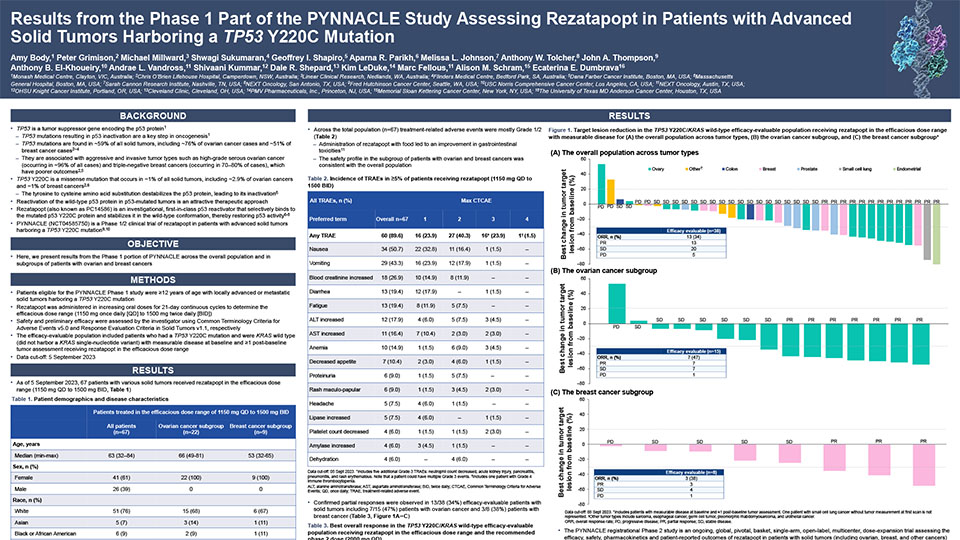
Updated Phase 1 results from the PYNNACLE Phase 1/2 study of PC14586, a selective p53 reactivator, in patients with advanced solid tumors harboring a TP53 Y220C mutation
Schram A, et al. Poster presentation at AACR-NCI-EORTC 2023, Boston, USA. October 11–15, 2023. Learn more.
Phase 1 analysis from the PYNNACLE Phase 1/2 study of PC14586 in the subgroup of patients with advanced ovarian cancer harboring a TP53 Y220C mutation
Schram A, et al. Oral presentation at SGO 2024, San Diego, USA. March 16–18, 2024. Learn more.
Biomarker testing can identify TP53 Y220C cancer
Biomarker testing can identify genomic abnormalities in your patient's tumor profile,1 including the TP53 Y220C mutation. This may provide information on whether an approved therapy or clinical study, such as the PYNNACLE study, could be an option for your patient.1
Reference: 1. American Cancer Society. Biomarker tests and cancer treatment. Available at: https://www.cancer.org/cancer/diagnosis-staging/tests/biomarker-tests.html. Accessed September 2024.
Presentations and Publications
Further information on clinical studies
Your patient can learn more about clinical studies with the National Cancer Institute, American Cancer Society, and European Medicines Agency.
If your patient is interested in taking part in a clinical trial listed on clinicaltrials.gov, the Leal Health service can help them find the right trial, for free. Share this link with your patient to provide them with more information.
To learn more about rezatapopt (also known as PC14586) in the PYNNACLE study, please visit clinicaltrials.gov.
Please contact the PMV Pharmaceuticals Clinical Study Information Center
+1 (609) 235-4038
clinicaltrials@pmvpharma.com
Rezatapopt is an investigational agent that has not been approved by the US FDA, EMA, or any other regulatory agency for the treatment of cancer.
Images do not depict actual trial participants.
MA-586-0035 October 2024